Here’s something that blew my mind: the monoclonal antibody market is expected to hit USD 450 billion by 2033 according to Evitria. That’s not just impressive numbers – it represents a complete game-changer in how we treat diseases, moving from carpet-bombing approaches to surgical precision strikes.
I’ve been following this field for years, and what amazes me most is how we’ve gone from crude laboratory experiments to treatments that can literally reprogram your immune system to fight cancer. Picture this: we started with antibodies that your body would attack like foreign invaders, and now we have treatments so precisely engineered that your immune system welcomes them as allies.
The journey hasn’t been smooth – it’s been filled with spectacular failures that taught us more than any successful experiment could have. But every setback brought us closer to treatments that work like molecular guided missiles.
Table of Contents
- The Generational Evolution: From Mouse Antibodies to Human-Engineered Therapeutics
- Understanding Antibody Architecture Through Function, Not Just Structure
- Target-Based Classification: How Mechanism Drives Clinical Success
- Why Making These Things Is So Damn Hard (And Why It Matters to You)
- Delivery Innovation: When How You Get It Matters as Much as What You Get
- The Regulatory Reality: Biosimilars, Orphan Drugs, and Market Access
- Connecting Antibody Precision to Personal Health Optimization
- Final Thoughts
TL;DR
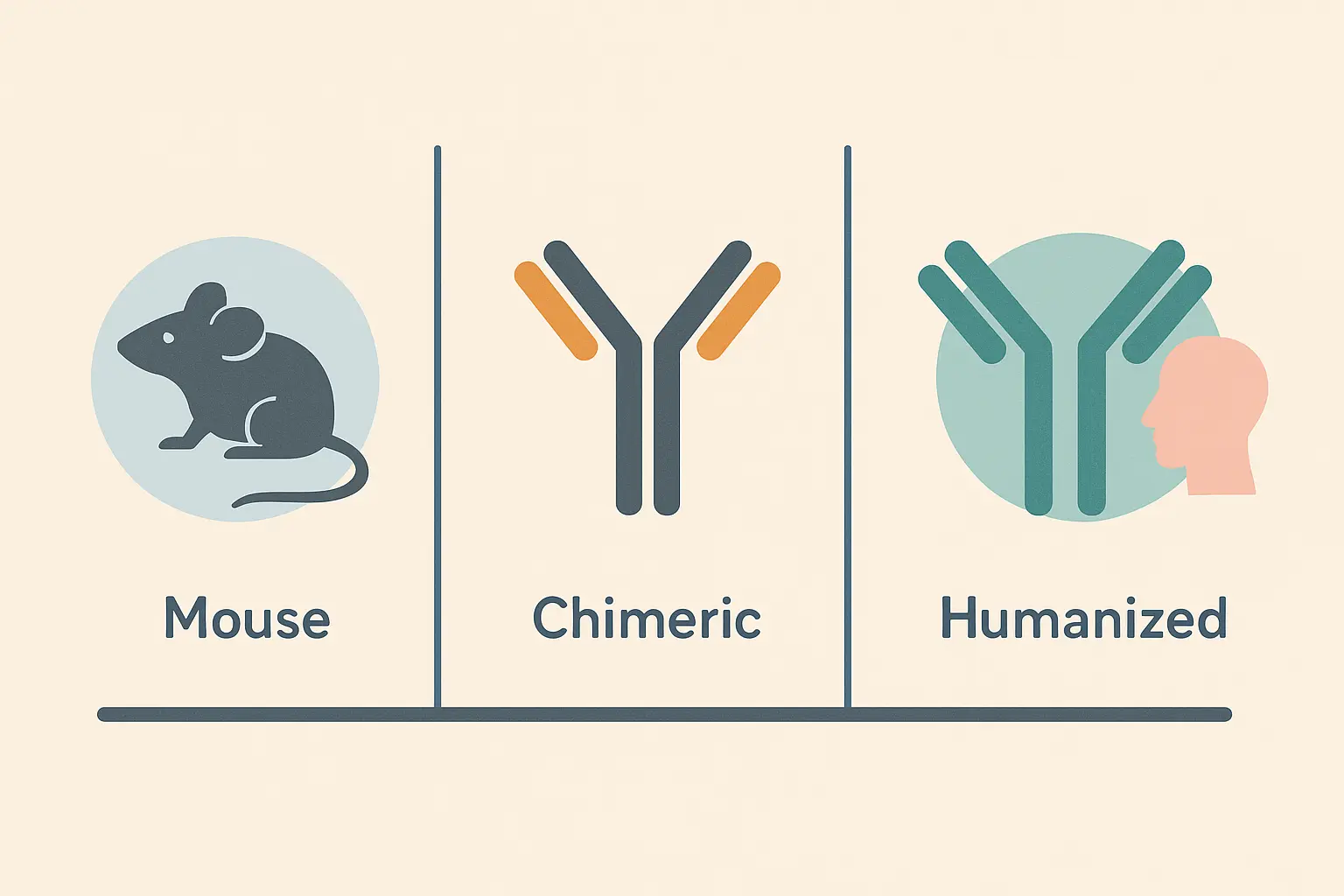
- Monoclonal antibodies evolved through distinct generations – from fully mouse-derived (your immune system hated them) to chimeric (70% human) to humanized (95% human) – each solving the problems that made patients sicker instead of better
- Here’s what actually matters: full-length monoclonal antibodies are like calling in the entire SWAT team, while fragments are like sending in a single sniper – each has its place depending on the job
- Forget the fancy names – checkpoint inhibitors unleash your natural immunity, receptor blockers stop growth signals, and antibody-drug conjugates are basically Trojan horses carrying poison directly to cancer cells
- Manufacturing complexity directly impacts whether you can actually get these treatments – making bispecific antibodies is like solving a Rubik’s cube blindfolded, while biosimilars face regulatory hurdles that make generic drugs look simple
- The revolution isn’t just in the lab – subcutaneous formulations mean cancer patients can get treatments at home instead of spending all day hooked up to an IV
- The same precision medicine principles that determine whether you get trastuzumab or pembrolizumab for cancer should apply to your daily health optimization – yet most of us are still taking generic supplements like it’s 1995
Understanding the types of monoclonal antibodies isn’t about memorizing categories – it’s about seeing how each format solves real problems that were killing patients.
The Generational Evolution: From Mouse Antibodies to Human-Engineered Therapeutics
Think of monoclonal antibody development like the evolution of smartphones. The first generation proved the concept but had major flaws, each subsequent generation fixed the biggest problems while creating new challenges, and now we have sophisticated devices that would seem like magic to someone from the 1990s.
The development of monoclonal antibodies follows this same pattern – from first-generation mouse antibodies that your immune system attacked on sight, to second-generation chimeric antibodies that found the sweet spot between effectiveness and acceptance, to third-generation humanized antibodies that are so sophisticated your body treats them like old friends.
I’ve watched this field transform from a fascinating laboratory curiosity into one of medicine’s most powerful therapeutic platforms. The story isn’t just about scientific progress – it’s about learning from failures that broke researchers’ hearts and systematically solving problems that seemed impossible.
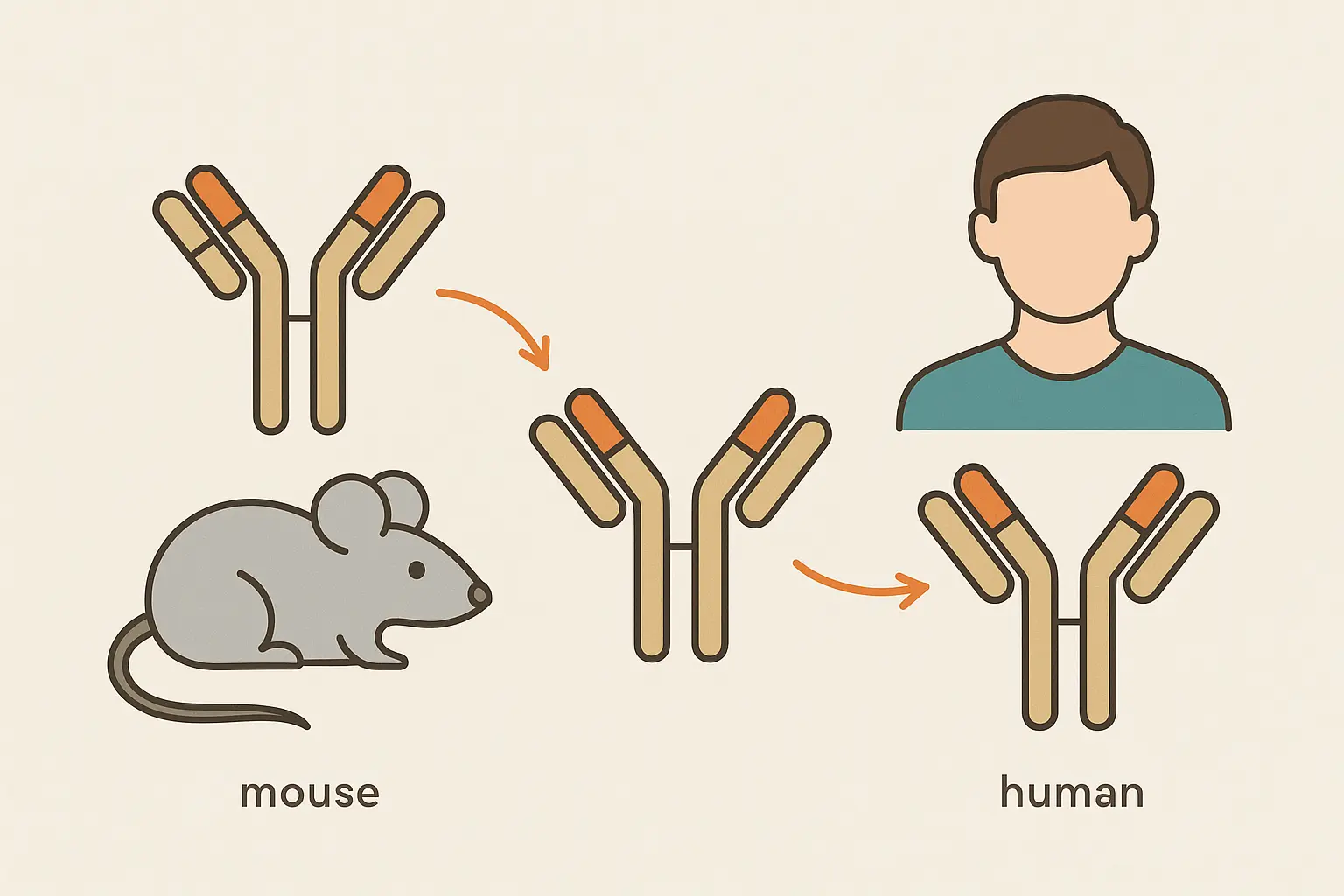
First Generation: When Mouse Antibodies Taught Us Hard Lessons
The original monoclonal antibodies were like borrowing a friend’s immune system to fight your battles. These fully mouse-derived mab products could target diseases with laser precision, but there was one massive problem – your immune system treated them like invading aliens.
Imagine getting the perfect weapon to fight cancer, only to discover that your own body’s security system would destroy it before it could do its job. That’s exactly what happened with first-generation mouse antibodies.
The HAMA Response: When Your Immune System Becomes the Enemy
Here’s where things got heartbreaking. The first dose of a mouse monoclonal antibody might work beautifully – patients would see their tumors shrink, their symptoms improve, hope would return. But by the second or third dose, their bodies had figured out how to neutralize the treatment.
Every time you received a mouse monoclonal antibody treatment, your immune system got better at recognizing and eliminating it. It was like training your body’s security force to attack the very medicine designed to save you.
The human anti-mouse antibody response created a cruel therapeutic window that narrowed with each dose. Patients and their families would watch treatments that initially seemed miraculous become progressively less effective, not because the cancer was getting stronger, but because their own immune systems were getting better at sabotaging their treatment.
I remember talking to a researcher who spent three years perfecting a mouse antibody, only to watch it fail spectacularly in the second human trial. The patient’s immune system attacked the treatment so aggressively that they ended up sicker than when they started. That failure taught us more about humanizing antibodies than any successful experiment could have.
Manufacturing Nightmares That Shaped Modern Production
Working with hybridomas was like trying to run a pharmaceutical company with a bunch of temperamental artists. Each cell line had its own personality – some produced mabs consistently, others would suddenly go on strike for no apparent reason, and predicting which ones would succeed was like reading tea leaves.
Picture this: You’re a biotech company that just spent millions developing a breakthrough antibody. But when you try to make it at scale, the cells decide to stop producing for no apparent reason. This isn’t science fiction – this was Tuesday in the early antibody world.
Researchers from that era describe hybridoma production as part science, part art, and part prayer. You’d create hundreds of cell lines, hoping a few would produce enough monoclonal antibody for clinical testing. The successful ones became precious resources, carefully maintained like rare orchids that might die if you looked at them wrong.
Second Generation: The Chimeric Compromise That Changed Everything
The breakthrough came when researchers realized they didn’t need to humanize the entire mab – just the parts that were causing the biggest problems. Think of it like replacing the engine in a foreign car with a domestic one – you keep what works (the mouse targeting system) but swap out what causes trouble (the parts your immune system recognizes as foreign).
By keeping the mouse variable regions (the business end that finds targets) and replacing the constant regions with human sequences, they created monoclonal antibodies that were foreign enough to work but human enough to avoid getting kicked out of the party.
| Generation | Human Content | Naming Convention | What This Means for Patients | Example Drug |
|---|---|---|---|---|
| First (Mouse) | 0% | -omab | Body attacks treatment after first few doses | Muromonab-CD3 |
| Second (Chimeric) | ~70% | -ximab | Can be given repeatedly with less rejection | Rituximab |
| Third (Humanized) | ~95% | -zumab | Body accepts it like a natural antibody | Trastuzumab |
| Fourth (Fully Human) | 100% | -umab | Lowest chance of immune reactions | Adalimumab |
Why 70% Human Became the Magic Number
The 70/30 split wasn’t chosen randomly – it was the result of countless experiments trying to find the sweet spot. Too much mouse content and your immune system would revolt. Too little and you’d lose the targeting precision that made the treatment work in the first place.
This taught us something crucial about medical innovation: sometimes the solution isn’t adding more sophisticated features, but rather removing the problematic ones while preserving what works. It’s like editing a manuscript – knowing what to cut is as important as knowing what to keep.
Rituximab: The Game-Changer That Proved the Concept
When rituximab succeeded in treating B-cell lymphomas, it didn’t just prove that chimeric mabs worked – it revealed something revolutionary. These antibodies weren’t just blocking disease processes; they were actually recruiting patients’ own immune cells to join the fight.
Picture rituximab as a molecular bouncer that not only identifies troublemakers but also calls in backup. It would bind to cancer cells and essentially paint a target on them, telling your natural killer cells and macrophages: “These are the bad guys – take them out.”
This was huge because it meant monoclonal antibodies weren’t just passive blockers – they were active recruiters of your immune system. The mab became like a general rallying troops for a coordinated attack on cancer.
Real Patient Story: A 65-year-old man with relapsed non-Hodgkin lymphoma had failed multiple rounds of chemotherapy. His oncologist was running out of options when rituximab became available. Within months of starting treatment, his tumors began shrinking. Five years later, he was playing golf and planning his retirement. The monoclonal antibody had essentially trained his immune system to recognize and eliminate cancer cells his body had been ignoring.
Third Generation: Humanized Precision Engineering
Here’s where things got really sophisticated. Instead of keeping entire mouse variable regions, researchers learned to identify just the tiny loops that actually touch the target – the complementarity-determining regions (CDRs) – and transplant only those sequences into fully human monoclonal antibody frameworks.
Think of it like performing surgery on a Swiss watch while wearing oven mitts. Scientists had to identify exactly which tiny pieces were essential – down to individual atoms – without breaking the whole mechanism.
CDR Grafting: Molecular Surgery at the Amino Acid Level
CDR grafting required understanding mab structure at a level of detail that seemed impossible when the field started. Researchers had to map exactly which amino acids were essential for target binding and which ones could be swapped out for human sequences without losing the antibody’s effectiveness.
This wasn’t trial and error – it was precision engineering that required sophisticated computer modeling, detailed understanding of protein folding, and the kind of patience that would drive most people crazy. The techniques developed for monoclonal antibody humanization ended up advancing the entire field of protein engineering.
When Simple Transplantation Failed: Framework Engineering Challenges
Here’s where things got complicated. Simply transplanting mouse CDRs into human frameworks was like trying to transplant a Formula 1 engine into a family sedan – the parts might fit, but they wouldn’t work together properly.
Many early humanization attempts failed because the binding activity would disappear, even though researchers had carefully preserved all the sequences that directly contacted the target. It was incredibly frustrating – like following a recipe exactly but ending up with something completely different.
The problem was that CDR shape depends on the surrounding framework, kind of like how a suspension bridge’s cables depend on the towers for support. Certain amino acids in the framework regions influence how the CDRs fold and position themselves, even though they never touch the target directly.
This led to the discovery of “Vernier zone” residues – framework positions that fine-tune CDR positioning like adjusting the focus on a microscope. This breakthrough revolutionized how we think about monoclonal engineering and pushed the field toward even more sophisticated approaches.
Understanding Antibody Architecture Through Function, Not Just Structure
Forget about where antibodies came from – what matters is what they’re designed to do. Each mab format represents different trade-offs between therapeutic properties like how long they last in your body, how well they penetrate tissues, and whether they recruit your immune system.
Think of it like choosing the right tool for a job. You wouldn’t use a sledgehammer to hang a picture frame, and you wouldn’t use a screwdriver to demolish a wall. Different antibody formats excel in different therapeutic situations.
Therapeutic antibodies represent the fastest-growing class of biological drugs approved for clinical use, demonstrating their significant impact across diverse disease areas according to News Medical. This growth reflects our increasing understanding of how to match the right antibody format to specific therapeutic challenges.
Full-Length Antibodies: Evolution’s Optimized Design
There’s a reason full-length monoclonal antibodies dominate the therapeutic landscape – they’re essentially pre-optimized by millions of years of evolution. Mother Nature spent eons perfecting the standard antibody design, and it shows.
The standard antibody structure isn’t just a default choice; it’s specifically designed for optimal performance in the human body. Think of it as the Swiss Army knife of the immune system – versatile, reliable, and effective across a wide range of situations.
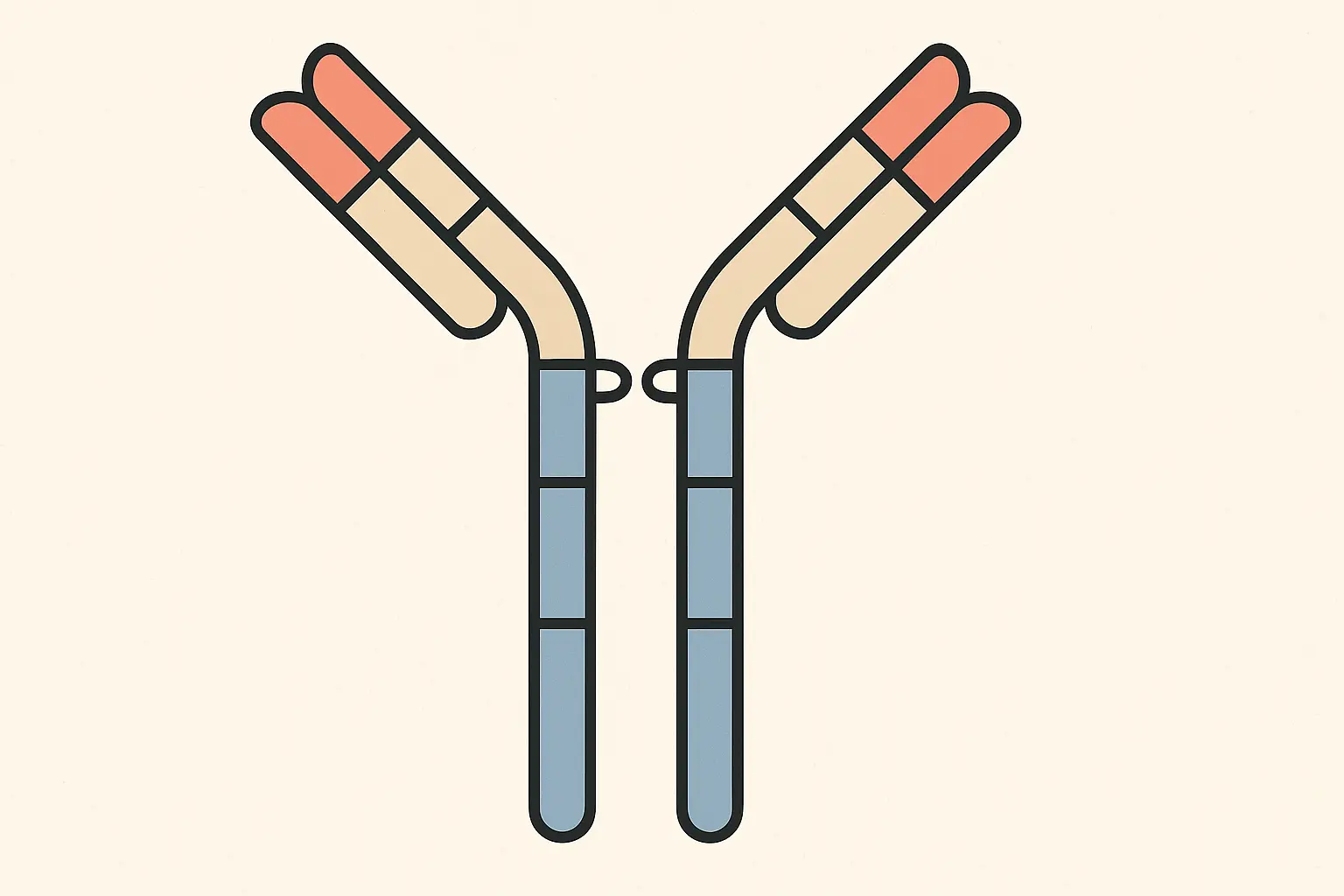
The Fc Region: Your Immune System’s Recognition Signal
The Fc region is where the magic happens for immune system recruitment. This isn’t just structural scaffolding – it’s an active signaling platform that tells your immune cells what to do when they encounter an antibody-coated target.
When your natural killer cells or macrophages see a monoclonal antibody bound to a cell surface, they recognize the Fc region and interpret it as a “destroy this target” signal. The mab essentially acts as a molecular flag that marks cells for elimination.
Modern antibody engineering includes deliberate Fc modifications. Some mabs are designed with enhanced immune-recruiting activity for more aggressive attacks on cancer cells, while others have “silenced” Fc regions to avoid unwanted immune activation in sensitive tissues. It’s like having
Modern antibody engineering includes deliberate Fc modifications. Some mabs are designed with enhanced immune-recruiting activity for more aggressive attacks on cancer cells, while others have “silenced” Fc regions to avoid unwanted immune activation in sensitive tissues. It’s like having a volume control for your immune response.
Bivalent Binding: When Two Sites Create Exponential Strength
Having two binding sites doesn’t just double the binding strength – it creates something called avidity that can be exponentially more powerful. When both binding sites engage simultaneously, the monoclonal antibody becomes incredibly difficult to shake loose from its target.
Think of it like molecular velcro. One hook might come loose easily, but when hundreds of hooks engage simultaneously, the bond becomes remarkably strong. This creates an interesting selectivity advantage that’s crucial for therapeutic success.
Targets with high surface density (like overexpressed receptors on cancer cells) can engage both binding sites simultaneously, leading to very strong binding. Targets with low expression levels might only engage one site at a time, resulting in weaker binding that’s more easily overcome.
The mab essentially becomes more selective for the cells you actually want to target – the ones overexpressing the disease-related proteins.
Antibody Fragments: Precision Tools for Specific Jobs
Sometimes you don’t want all the features that come with a full-length monoclonal antibody. Maybe you need to penetrate deep into solid tumors where large antibodies can’t reach, or you want to avoid immune system recruitment, or you’re targeting something inside cells.
Antibody fragments represent deliberate trade-offs – giving up properties like long circulation time to gain advantages like rapid tissue penetration or reduced immune reactions.
Fab and F(ab’)2: The Original Proof of Concept
The original antibody fragments proved you could separate binding activity from immune system recruitment. Fab fragments retain full binding specificity but lose the ability to call in immune backup or activate complement.
For certain applications, this is exactly what you want. Rapid clearance becomes a feature rather than a bug when you’re doing imaging studies where you want contrast agents to clear quickly, or delivering toxins where you want to minimize off-target exposure.
Think of Fab fragments as surgical strikes rather than calling in air support – precise, targeted, and gone before they can cause collateral damage.
Single-Chain Variable Fragments: Minimal Functional Units
scFv fragments represent the absolute minimum functional antibody – just the targeting components connected by a flexible linker. At about one-sixth the size of full monoclonal antibodies, they can go places that larger antibodies simply can’t reach.
The small size enables penetration into solid tumors where large mabs can’t achieve therapeutic concentrations. They can cross certain biological barriers that exclude larger proteins, and when engineered properly, they can even access targets inside cells.
But this minimalism comes with challenges. scFv fragments often suffer from stability issues and tend to clump together, requiring sophisticated protein engineering to make them suitable for therapeutic use. It’s like building a race car – you can make it incredibly fast, but you sacrifice comfort and reliability.
| Antibody Format | Size | How Long It Lasts | Where It Can Go | What It Can Do | Best Use |
|---|---|---|---|---|---|
| Full IgG | Large | Weeks | Bloodstream, organs | Recruit immune system | Cancer treatment |
| Fab | Medium | Hours | Good tissue penetration | Block targets only | Imaging, neutralization |
| F(ab’)2 | Medium-large | Days | Good tissue access | Limited immune recruitment | Diagnostics |
| scFv | Tiny | Minutes-hours | Everywhere, even tumors | Pure targeting | Tumor penetration |
Target-Based Classification: How Mechanism Drives Clinical Success
Here’s what really matters when understanding antibody types – forget the fancy structural names and focus on what they actually do to fight disease. The target and mechanism directly determine whether a treatment will work for you, what side effects you might experience, and how your doctor will monitor your progress.
Think of it this way: you wouldn’t choose a car based on what factory made it – you’d choose based on whether you need a sports car, a pickup truck, or a minivan. The same logic applies to monoclonal antibodies.
Immune Checkpoint Modulators: Taking the Brakes Off Your Anti-Cancer Immunity
Checkpoint inhibitors represent a completely different philosophy of cancer treatment. Instead of directly attacking cancer cells like traditional chemotherapy, these monoclonal antibodies remove the molecular “stop” signs that prevent your immune system from doing what it’s naturally designed to do – eliminate abnormal cells.
It’s like having a guard dog that’s been trained to never bite intruders. Checkpoint inhibitors essentially remove that training and let your immune system do its job.
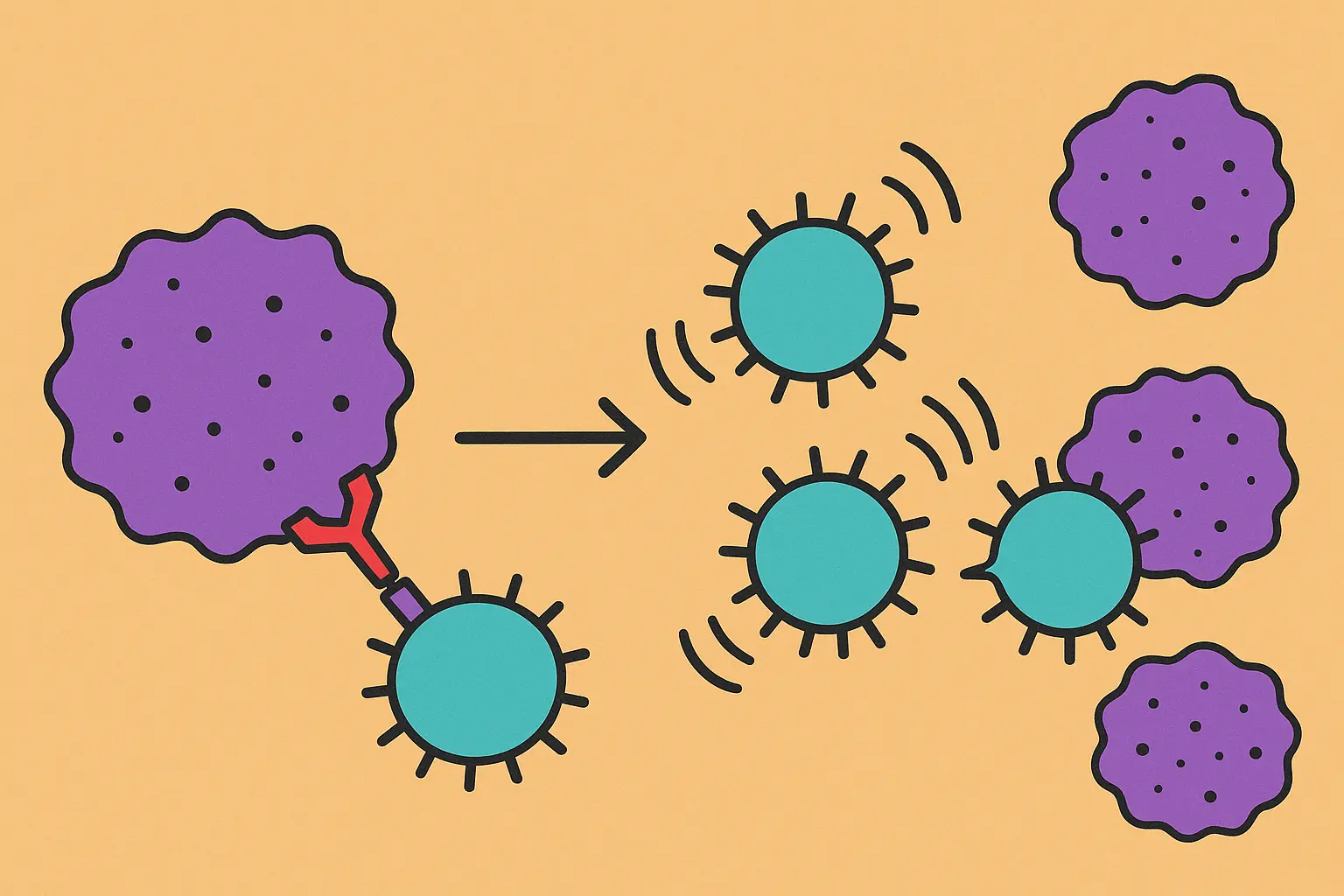
PD-1/PD-L1 Pathway: The Breakthrough That Rewrote Cancer Treatment
The PD-1/PD-L1 pathway is essentially cancer cells’ way of wearing a “don’t shoot me” vest. Cancer cells display PD-L1 on their surface, which binds to PD-1 on your T-cells and tells them to stand down. It’s molecular camouflage that lets cancer hide in plain sight.
PD-1 inhibitors work like removing those fake police badges. Suddenly, your T-cells can see cancer cells for what they really are and attack them accordingly.
When pembrolizumab was approved based on tumor genetics rather than where the cancer was located, it fundamentally changed oncology. For the first time, we had a treatment that worked based on molecular fingerprints rather than anatomical location. A colon cancer with the right molecular signature might respond better to pembrolizumab than a melanoma without it.
This was revolutionary because it meant we needed to stop thinking about cancer as “breast cancer” or “lung cancer” and start thinking about it as diseases defined by their molecular characteristics. The mab essentially taught us that location matters less than biology.
CTLA-4 Inhibition: The Pioneer That Proved Unleashing Immunity Could Work
Ipilimumab was the first checkpoint inhibitor to succeed clinically, and it taught us both the incredible promise and the serious risks of removing immune system brakes. Some melanoma patients achieved complete remissions that lasted for years – something virtually unheard of with advanced cancer.
But ipilimumab also showed us that taking the brakes off your immune system can cause it to attack healthy tissues. The same mechanism that allows T-cells to destroy cancer can also cause them to attack your colon, liver, or endocrine glands.
It was like giving someone a loaded gun to defend their house but discovering they might accidentally shoot the neighbors too. The power was undeniable, but it required incredible precision in patient selection and monitoring.
Real Patient Impact: A 45-year-old teacher with metastatic melanoma was given six months to live when ipilimumab became available. The first few treatments made her feel worse – fatigue, diarrhea, rashes. Her family wondered if the cure was worse than the disease. But six months later, her scans showed no detectable cancer. Seven years later, she’s still cancer-free and back to teaching full-time.
Cell Surface Receptor Antagonists: Precision Blocking Strategies
Monoclonal antibodies targeting cell surface receptors work by intercepting the molecular text messages that tell cells to grow and divide. By binding to receptors on cell surfaces, they prevent growth signals from getting through – like jamming a radio frequency.
Trastuzumab: The Success Story That Defined Targeted Therapy
Trastuzumab transformed breast cancer treatment by proving that precision targeting could be as effective as chemotherapy while being far more tolerable. Instead of poisoning all rapidly dividing cells, trastuzumab specifically targets cancer cells addicted to HER2 signaling.
The key insight was that some breast cancers are essentially junkies for HER2 growth signals. By blocking this single pathway, you could effectively starve these tumors while leaving healthy tissues largely unaffected.
HER2-Positive Treatment Success: A 52-year-old nurse discovered a lump during a routine self-exam. Biopsy confirmed invasive ductal carcinoma, and further testing revealed 3+ HER2 expression. Instead of the aggressive chemotherapy regimen her sister had endured five years earlier, she received trastuzumab-based therapy. The side effects were manageable – some fatigue and joint aches – nothing like the devastating nausea and hair loss she’d feared. Five years later, she’s disease-free and back to working full shifts in the ICU.
When Cancer Fights Back: How Resistance Drives Innovation
Here’s the frustrating reality about cancer – it’s incredibly adaptable. When you block one growth pathway, cancer cells often find alternative routes to keep proliferating. It’s like water finding a way around a dam.
This constant evolutionary pressure has driven the development of combination therapies and next-generation mabs designed to stay one step ahead of cancer’s adaptation strategies.
The evolution from trastuzumab to T-DM1 (trastuzumab emtansine) perfectly illustrates this arms race. When cancer cells developed resistance to receptor blocking, researchers created an antibody-drug conjugate that uses the same targeting mechanism to deliver a potent toxin directly to resistant cells. If you can’t starve them, poison them – but do it precisely.
Antibody-Drug Conjugates: Guided Missile Therapeutics
Antibody-drug conjugates represent the ultimate precision weapon in cancer treatment. They combine the targeting accuracy of monoclonal antibodies with the killing power of chemotherapy drugs that would be too toxic to give systemically.
Think of ADCs as Trojan horses – they look harmless on the outside, but they’re carrying something deadly that only gets released once they’re inside enemy territory.
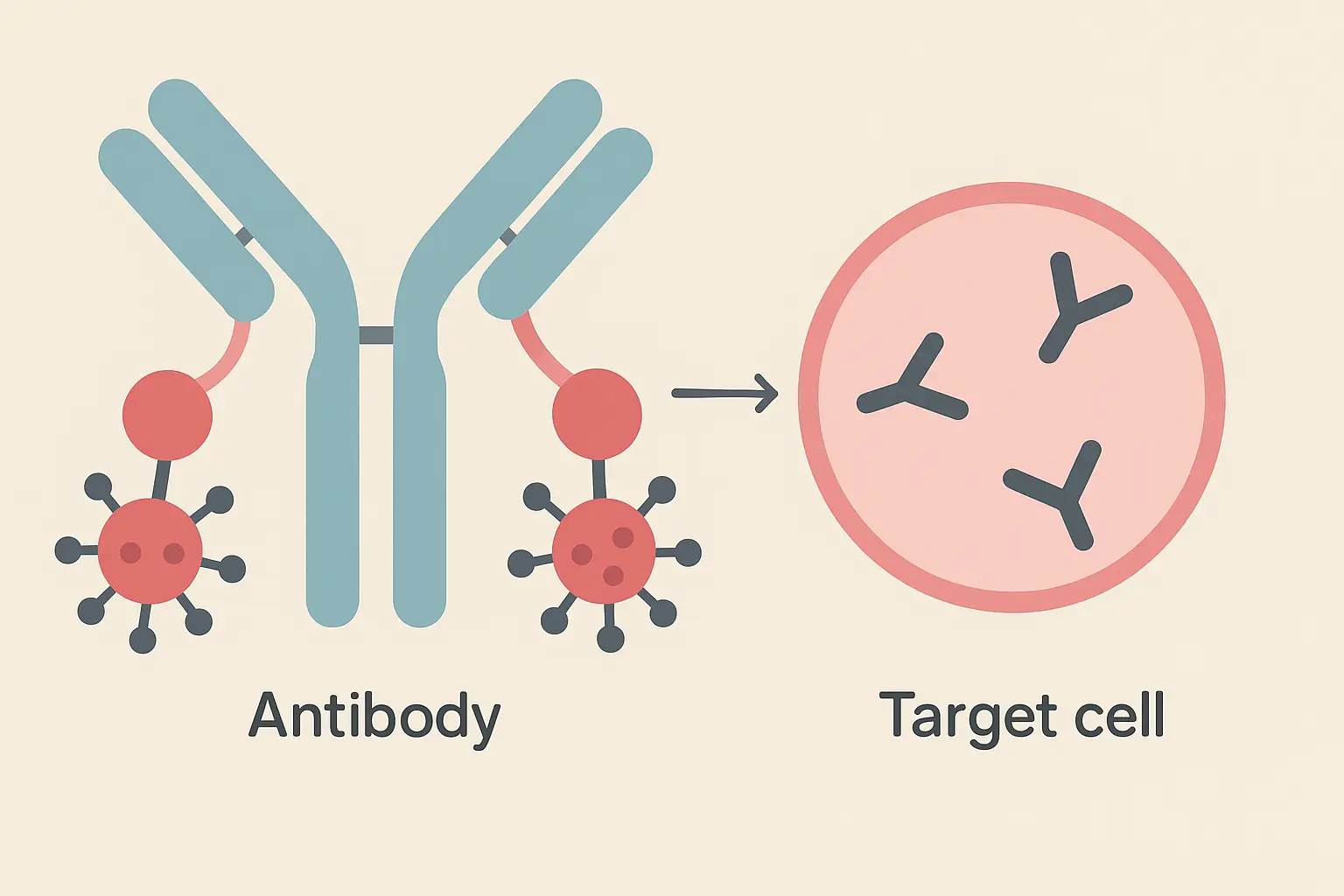
The Trojan Horse Strategy: Smuggling Toxins Past Cellular Defenses
The concept sounds simple: attach a powerful toxin to a monoclonal antibody that specifically recognizes cancer cells. The mab acts as a molecular delivery truck, carrying poison directly to its intended target while avoiding healthy neighborhoods.
But the execution is incredibly complex. The linker connecting antibody to drug must remain stable during weeks of circulation but then release the drug efficiently once inside the target cell. It’s like designing a time-release capsule that only opens under very specific conditions.
Some ADC payloads can actually leak out of the target cell and kill nearby cells that don’t express the target antigen. This “bystander effect” can be therapeutically beneficial by eliminating cancer cells that have lost target expression, but it can also cause toxicity in adjacent normal tissues.
Payload Selection: Choosing the Right Weapon for Molecular Delivery
The drugs used in ADCs aren’t your typical chemotherapy agents. They need to be incredibly potent because only tiny amounts reach each target cell, and they must remain stable when attached to mabs circulating in your bloodstream for weeks.
Microtubule inhibitors cause cell cycle arrest by preventing proper chromosome separation during cell division – imagine trying to divide a cell when its internal scaffolding is frozen in place. DNA-damaging agents directly break DNA strands, triggering cellular suicide pathways.
Each payload class creates different therapeutic profiles. Some cause cells to die quickly but messily, while others trigger clean, programmed cell death. The choice depends on the cancer type, the target expression pattern, and what side effects are acceptable.
Why Making These Things Is So Damn Hard (And Why It Matters to You)
The sophistication required to manufacture different types of monoclonal antibodies directly determines which treatments actually reach patients and how much they cost. Manufacturing complexity isn’t just a technical challenge – it’s a barrier that can mean the difference between life and death for patients who need these treatments.
Here’s the brutal reality: a breakthrough antibody that can’t be manufactured reliably and affordably might as well not exist. The most elegant scientific discovery means nothing if it can’t make it from the lab to the patient’s bedside.
Monoclonal antibodies (mAbs) 2E4 and AE6 targeting 36 indole-type and indazole-type SCs and their metabolites with IC 50 ranging from 0.14 to 85.28 ng/mL were prepared demonstrates the precision achievable in modern antibody development according to recent research published in Analytical Chemistry. This level of precision requires manufacturing processes that work like Swiss clockwork, adding layers of complexity that directly impact both cost and patient access.
Bispecific Antibodies: Engineering Dual Specificity
Bispecific monoclonal antibodies represent the next evolution in antibody engineering, but they come with manufacturing headaches that have historically limited their development. When you try to make a mab with two different binding specificities, the production cells behave like confused teenagers – they don’t know which instructions to follow.
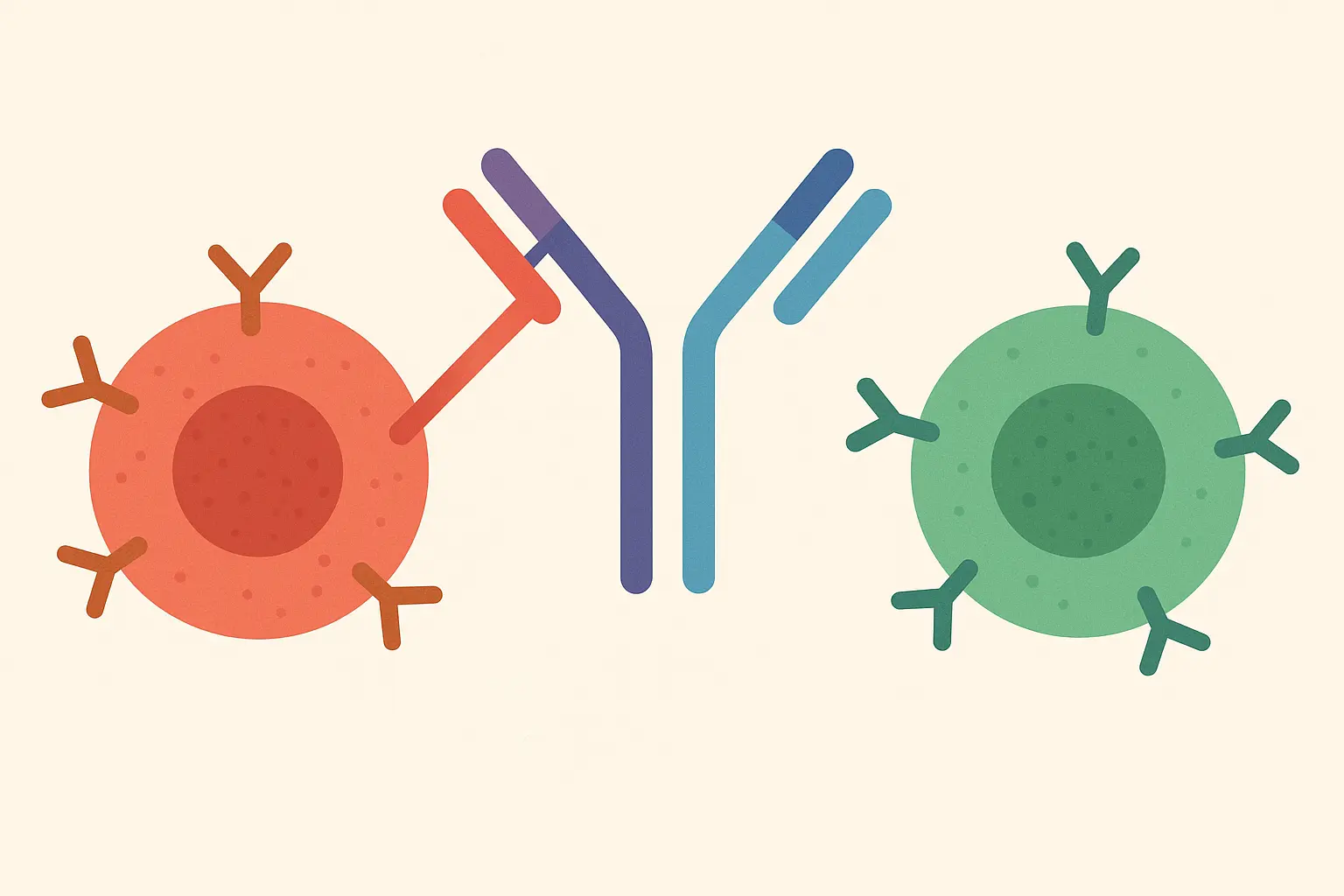
The Chain Pairing Problem: When Biology Doesn’t Cooperate
When you introduce four different antibody chains into a cell (two different heavy chains and two different light chains), the cell randomly assembles them into various combinations. It’s like trying to assemble two different jigsaw puzzles when all the pieces are mixed together in the same box.
Out of all the possible combinations, only one produces the desired bispecific mab – the rest are either completely useless or have unintended specificities that could be dangerous. Most of what the cells produce ends up being expensive garbage.
Technologies like “knobs-into-holes” solve this by engineering complementary mutations that force the correct chains to pair together. One heavy chain gets a “knob” mutation while its partner gets a corresponding “hole” mutation, ensuring they preferentially stick together rather than forming random combinations. It’s molecular engineering at its finest, but it requires incredible precision.
T-Cell Engagers: Bringing Your Immune System to the Fight
T-cell engagers work by physically connecting your cytotoxic T-cells to cancer cells, essentially turning every T-cell in your body into a tumor-seeking missile. One end binds to CD3 on T-cells, while the other end binds to a tumor-associated antigen.
When this connection forms, it creates an immunological handshake that activates the T-cell to kill the cancer cell. The problem is that this activation can be so powerful that it triggers massive inflammatory responses that can be life-threatening.
Blinatumomab Treatment Reality: A 45-year-old patient with relapsed leukemia receives blinatumomab through continuous IV infusion. The first dose starts incredibly low – just 9 μg/day – because even tiny amounts can cause overwhelming immune activation. The patient needs hospitalization for monitoring, with emergency medications on standby in case their immune system goes haywire. It’s powerful medicine that requires incredible caution.
Biosimilar Antibodies: The Generic Challenge That Isn’t Really Generic
Here’s something most people don’t understand: biosimilar monoclonal antibodies face a fundamentally different challenge than generic drugs. You can’t just copy the molecular structure and assume you’ll get the same therapeutic effect – mabs are living products of incredibly complex biological manufacturing processes.
Why Manufacturing Process Equals Product Identity
Small molecule drugs are like LEGO blocks – if you can build the same structure, you have the same product. Monoclonal antibodies are more like sourdough bread – the final product depends not just on the recipe, but on the starter culture, the temperature, the humidity, and dozens of other variables that affect how the living cells modify the antibodies.
Glycosylation patterns, protein folding, aggregation levels, and other modifications all influence how monoclonal antibodies behave in patients. These characteristics depend on everything from the specific cell line used for production to the temperature and pH of the culture medium.
This means biosimilar manufacturers can’t just reverse-engineer the mab sequence – they need to develop entirely new manufacturing processes that produce antibodies with similar characteristics to the original product. It’s like trying to recreate your grandmother’s famous recipe when she never wrote anything down and used “a pinch of this” and “a dash of that.”
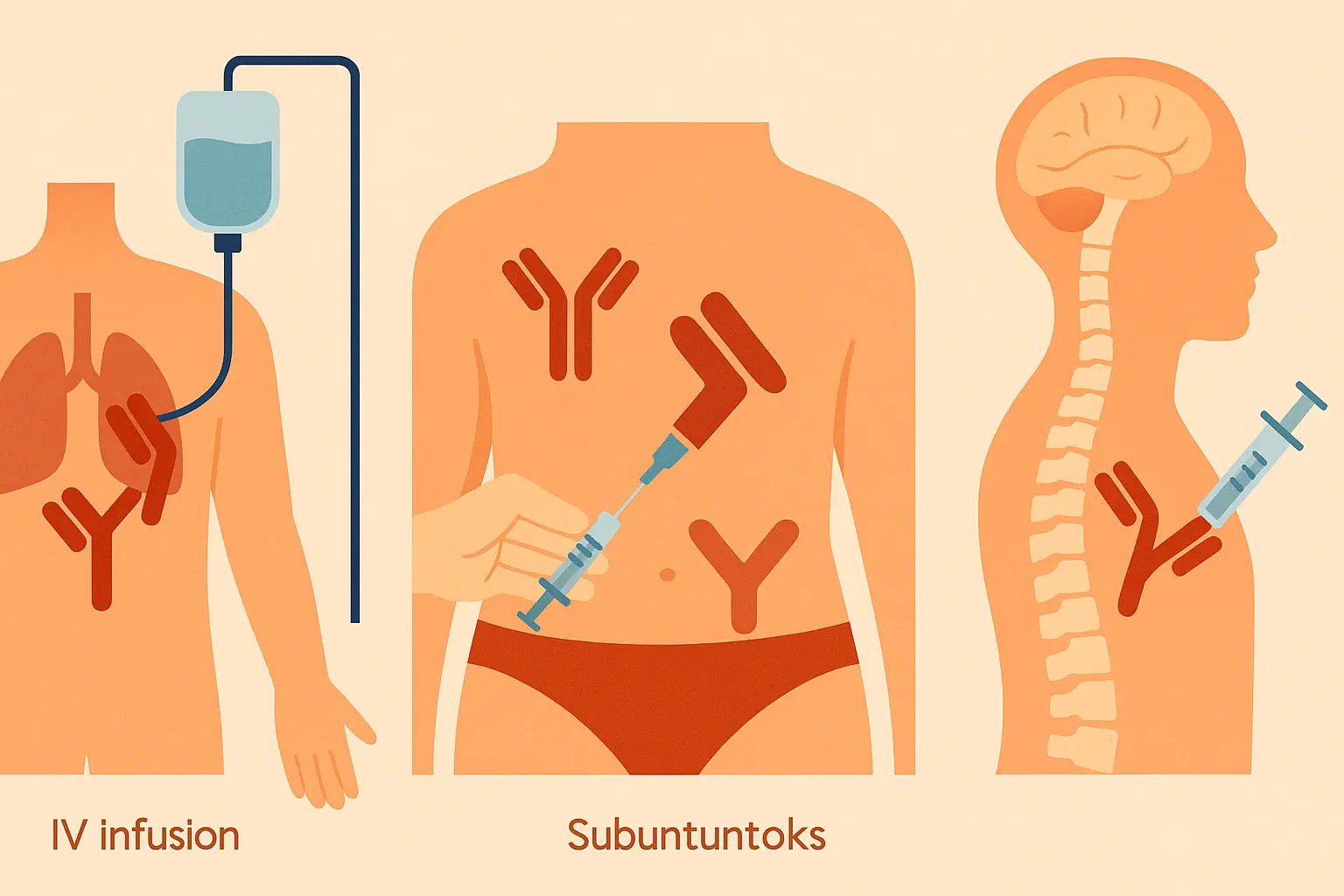
Delivery Innovation: When How You Get It Matters as Much as What You Get
The way monoclonal antibodies are formulated and delivered can be as therapeutically important as the mab itself. Delivery innovations have transformed the patient experience from day-long hospital visits to simple injections you can do at home.
All this engineering complexity boils down to one thing: a cancer patient can now get their treatment at home with a simple injection instead of spending eight hours hooked up to an IV in a hospital.
Subcutaneous Revolution: Bringing Hospital Treatments Home
The shift from intravenous to subcutaneous monoclonal antibody delivery represents more than just convenience – it’s fundamentally changed how patients experience treatment and manage their lives around therapy.
Instead of planning their entire week around a hospital visit, many patients can now give themselves their mab therapy while watching Netflix on their couch. The psychological impact of this change can’t be overstated.
Hyaluronidase: The Enzyme That Opened New Delivery Pathways
The challenge with subcutaneous antibody delivery was simple physics – therapeutic doses often required more fluid than you could comfortably inject under the skin. Hyaluronidase solved this by temporarily breaking down the tissue barriers that limit fluid absorption.
Think of hyaluronidase as creating temporary highways in your subcutaneous tissue that allow monoclonal antibody solutions to spread and absorb more efficiently. The effect is temporary and harmless, but it enables delivery of therapeutic doses that would otherwise require intravenous infusion.
Patient Experience Transformation: Sarah, a 58-year-old marketing executive with rheumatoid arthritis, used to block out entire days for her infusions. Between travel time, waiting room delays, and the actual infusion, it was an all-day affair that disrupted her work schedule and family life. Now she gives herself a subcutaneous injection every few weeks while having her morning coffee. The clinical effectiveness is identical, but her quality of life has dramatically improved.
Specialized Delivery Routes: Bypassing Biological Barriers
Some therapeutic targets are protected by biological barriers that prevent systemically administered monoclonal antibodies from reaching therapeutic concentrations. The blood-brain barrier is the most famous example, but other specialized compartments also require creative delivery approaches.
Intrathecal delivery involves injecting mabs directly into the cerebrospinal fluid, bypassing the blood-brain barrier entirely. This enables treatment of neurological conditions that would be impossible to address with standard injection routes.
The trade-off is complexity and risk – these delivery routes require specialized procedures and carry additional risks compared to standard injections. It’s like the difference between taking a pill and having brain surgery – both can be medically necessary, but one requires much more careful consideration.
The Regulatory Reality: Biosimilars, Orphan Drugs, and Market Access
The regulatory landscape for monoclonal antibodies reflects the complexity and diversity of these therapeutic agents. Different mab types follow different approval pathways, each designed to address specific challenges while ensuring patient safety and therapeutic efficacy.
Getting these treatments approved is like trying to prove your homemade cookies are exactly the same as your grandmother’s recipe – except grandma never wrote anything down, and people’s lives depend on getting it exactly right
Orphan Drug Antibodies: Specialized Pathways for Rare Diseases
Monoclonal antibodies targeting rare diseases operate under completely different commercial and regulatory logic than treatments for common conditions. When you’re dealing with a disease that affects fewer than 200,000 people in the entire United States, traditional development approaches simply don’t work.
For ultra-rare diseases affecting fewer than 1,000 patients globally, traditional clinical trial designs become impossible. You can’t randomize 500 patients to placebo when only 800 people worldwide have the condition. It’s like trying to conduct a political poll in a town with 12 residents.
This requires innovative approaches like natural history studies, single-arm trials, and regulatory flexibility that would be unthinkable for common diseases. The FDA essentially has to make decisions based on much smaller amounts of data, balancing the desperate need for treatments against the uncertainty that comes with limited clinical experience.
Real Orphan Drug Challenge: A biotech company develops a breakthrough antibody for a rare genetic disorder affecting 300 children worldwide. They spend $400 million on development, but the entire patient population couldn’t fill a high school auditorium. Even if every single patient uses the treatment, the company needs to charge hundreds of thousands of dollars per year just to break even. It’s a heartbreaking economic reality that puts life-saving treatments out of reach for many families.
Connecting Antibody Precision to Personal Health Optimization
Here’s something that should keep you up at night: the same precision medicine principles that determine whether you get trastuzumab or pembrolizumab for cancer should apply to your daily health optimization – yet most of us are still taking generic supplements like it’s 1995.
The evolution of monoclonal antibodies from broadly-acting treatments to precisely-targeted, biomarker-selected therapies mirrors what should be happening in preventive medicine and health optimization. The same principles that make modern mab therapy successful can revolutionize how you approach your individual health enhancement.
Biomarker-Driven Precision: From Cancer Treatment to Health Optimization
Modern oncologists wouldn’t dream of prescribing trastuzumab without confirming HER2 positivity, or using checkpoint inhibitors without understanding PD-L1 expression levels. This biomarker-driven approach ensures patients receive treatments most likely to benefit them while avoiding unnecessary exposure to ineffective therapies.
Yet when it comes to health optimization, most people are still using the medical equivalent of throwing spaghetti at the wall to see what sticks. Generic wellness approaches ignore individual variation in metabolism, genetics, and physiological needs.
Think about it: we’ve developed cancer treatments so precise that they target specific molecular pathways based on tumor genetics, but most people choose their vitamins based on marketing claims and internet reviews. The disconnect is staggering.
Just as monoclonal antibody therapy selection depends on tumor characteristics, optimal health interventions should be guided by individual physiological parameters. This might include genetic testing for supplement metabolism, hormone level assessment for optimization protocols, or microbiome analysis for personalized nutrition strategies.
Understanding individual hormonal health patterns becomes as critical for health optimization as determining antibody target expression is for cancer treatment.
Precision Health Application: Instead of taking a generic multivitamin, imagine getting a comprehensive analysis that reveals you have genetic variants affecting vitamin D metabolism, low baseline magnesium levels, and a microbiome profile that suggests specific probiotic strains. Your supplement regimen becomes as personalized as a cancer patient’s antibody therapy – targeted, monitored, and adjusted based on biomarker responses.
Continuous Monitoring: The Antibody Therapy Model for Health Enhancement
Successful monoclonal antibody therapy isn’t a “set it and forget it” approach. Patients receive regular monitoring for both therapeutic response and potential side effects, with treatment modifications based on individual outcomes. This dynamic approach recognizes that optimization is an ongoing process rather than a one-time intervention.
The sophisticated monitoring protocols used in antibody therapy mirror the comprehensive approach needed for peptide therapy optimization, where individual response patterns guide dose adjustments and treatment refinement.
Antibody Therapy Monitoring Protocol:
- Baseline biomarker assessment before treatment initiation
- Regular efficacy monitoring through imaging and lab work
- Continuous safety surveillance for adverse effects
- Dose modifications based on individual response patterns
- Long-term outcome tracking and treatment optimization
Five years from now, getting your personalized health optimization protocol should be as routine as getting your blood pressure checked. The same biomarker testing that guides cancer treatment will tell you exactly which supplements, exercises, and lifestyle changes will actually work for your unique biology.
Enov.one applies this same methodology to health optimization by integrating wearable data, periodic assessments, and treatment adjustments based on individual response patterns. The continuous monitoring protocols that ensure mab therapy safety and efficacy translate directly to ongoing health optimization strategies.
The sophisticated targeting mechanisms of modern monoclonal antibodies – from checkpoint inhibitors that modulate immune function to ADCs that deliver precise therapeutic payloads – demonstrate the power of personalized, mechanism-based interventions. This precision approach, applied to health optimization through personalized supplementation, hormone therapy, and lifestyle interventions, represents the natural evolution of precision medicine from disease treatment to health enhancement.
Final Thoughts
The journey through monoclonal antibody types reveals more than just scientific progress – it shows us how precision medicine principles can transform therapeutic outcomes when we stop accepting one-size-fits-all approaches and start treating each person as the unique biological individual they are.
What strikes me most about this evolution is how it mirrors the broader shift toward personalized medicine that should be happening in every aspect of healthcare. We’ve moved from crude mouse antibodies that your immune system rejected to sophisticated therapeutic agents selected based on individual biomarkers, genetic profiles, and disease characteristics.
When my colleague’s mother was diagnosed with HER2-positive breast cancer five years ago, trastuzumab turned what used to be a death sentence into a manageable condition. She’s now cancer-free and planning her daughter’s wedding. That transformation didn’t happen because of better generic treatments – it happened because precision medicine matched the right therapy to her specific cancer biology.
The manufacturing and delivery innovations that have made monoclonal antibody therapy more accessible – from subcutaneous formulations that enable home administration to biosimilar pathways that improve affordability – remind us that breakthrough science means nothing if it can’t reach the patients who need it.
The next time you’re tempted to buy that generic wellness supplement everyone’s raving about online, remember: the same precision that’s revolutionizing cancer treatment should be revolutionizing how you take care of yourself. Your biology is as unique as your fingerprint – isn’t it time your health plan reflected that?
As we continue pushing the boundaries of antibody engineering, the lessons learned from this remarkable therapeutic class will undoubtedly influence how we approach medicine broadly. The precision, personalization, and continuous optimization that define modern mab therapy represent the future of all therapeutic interventions – including the proactive health enhancement strategies that help us maintain optimal function throughout our lives.
The revolution isn’t just happening in cancer wards and research labs – it’s coming to how we think about health, prevention, and human optimization. The question isn’t whether precision medicine will transform healthcare – it’s whether you’ll be ready to embrace it when it does.
